Ammonium Nitrate
Dense & ANFO & Medical Ammonium Nitrate
Product Description
Ammonium Nitrate Introduction:
Chemical Formula: NH4NO3, simplified to N2H4O3
Ammonium nitrate is a chemical compound and commonly prepared by the reaction of ammonia with nitric acid. This is a typical acid-base reaction to give a salt (NH4NO3) as the product. NH3 + HNO3 → NH4NO3
Ammonium nitrate is readily water soluble. It is also highly hygroscopic, meaning that it readily absorbs water from the atmosphere and clumps up. It is not particularly reactive and is fairly stable. It decomposes at high temperatures (over 200 °C) to form nitrous oxide (laughing gas) and water vapor. Because solid ammonium nitrate can undergo explosive decomposition when heated in a confined space, government regulations have been imposed on its shipment and storage.
Physical Forms:
Ammonium nitrate is commercially available both as a colorless crystalline solid or bead-like powder and processed into Prills for specific applications. Soluble in water.
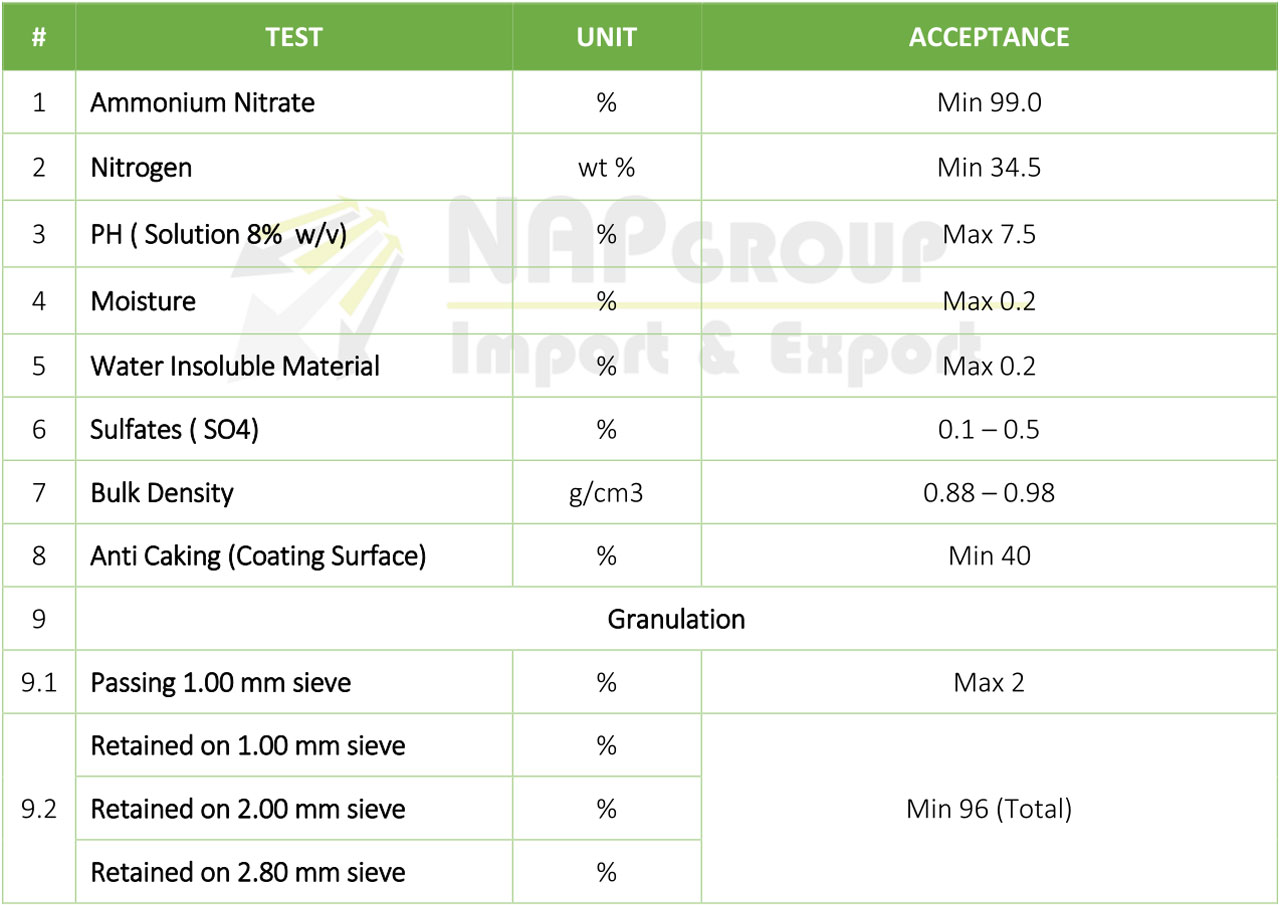
• Ammonium nitrate based fertilizers is a white solid in the form of Prills. Soluble in water.
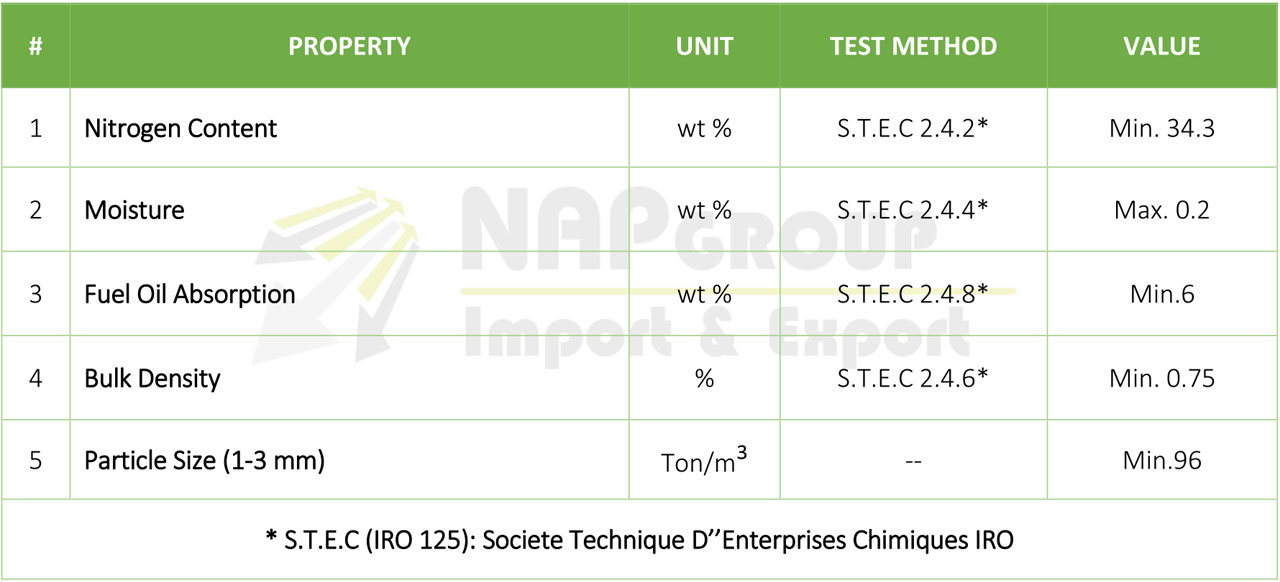
• Ammonium nitrate based explosives is a powder. Readily explodes if heated. Difficult to explode by shock. Toxic nitrogen oxide fumes may be released during combustion. Primary hazard is blast of an instantaneous explosion, not flying projectiles or fragments.
USES:
It is predominantly used in agriculture as a high-nitrogen fertilizer. Its other major use is as a component of explosive mixtures used in mining, quarrying, and civil construction, as described below:
• Fertilizer
Ammonium nitrate is an important fertilizer with the NPK rating 34-0-0 (34% nitrogen). It is less concentrated than urea (46-0-0), giving ammonium nitrate a slight transportation disadvantage. Ammonium nitrate’s advantage over urea is that it is more stable and does not rapidly lose nitrogen to the atmosphere. During warm weather it is best to apply urea soon before rain is expected or to cover it with soil to minimize nitrogen loss.
• Explosives
Ammonium nitrate is not, on its own, an explosive, but it readily forms explosive mixtures with varying properties when combined with primary explosives such as azides or with fuels such as aluminum powder or fuel oil.
• Mixture with fuel oil
ANFO is a mixture of 94% ammonium nitrate (“AN”) and 6% fuel oil (“FO”) widely used as a bulk industrial explosive. It is used in coal mining, quarrying, metal mining, and civil construction in undemanding applications where the advantages of ANFO’s low cost and ease of use matter more than the benefits offered by conventional industrial explosives, such as water resistance, oxygen balance, high detonation velocity, and performance in small diameters.
• Other USES:
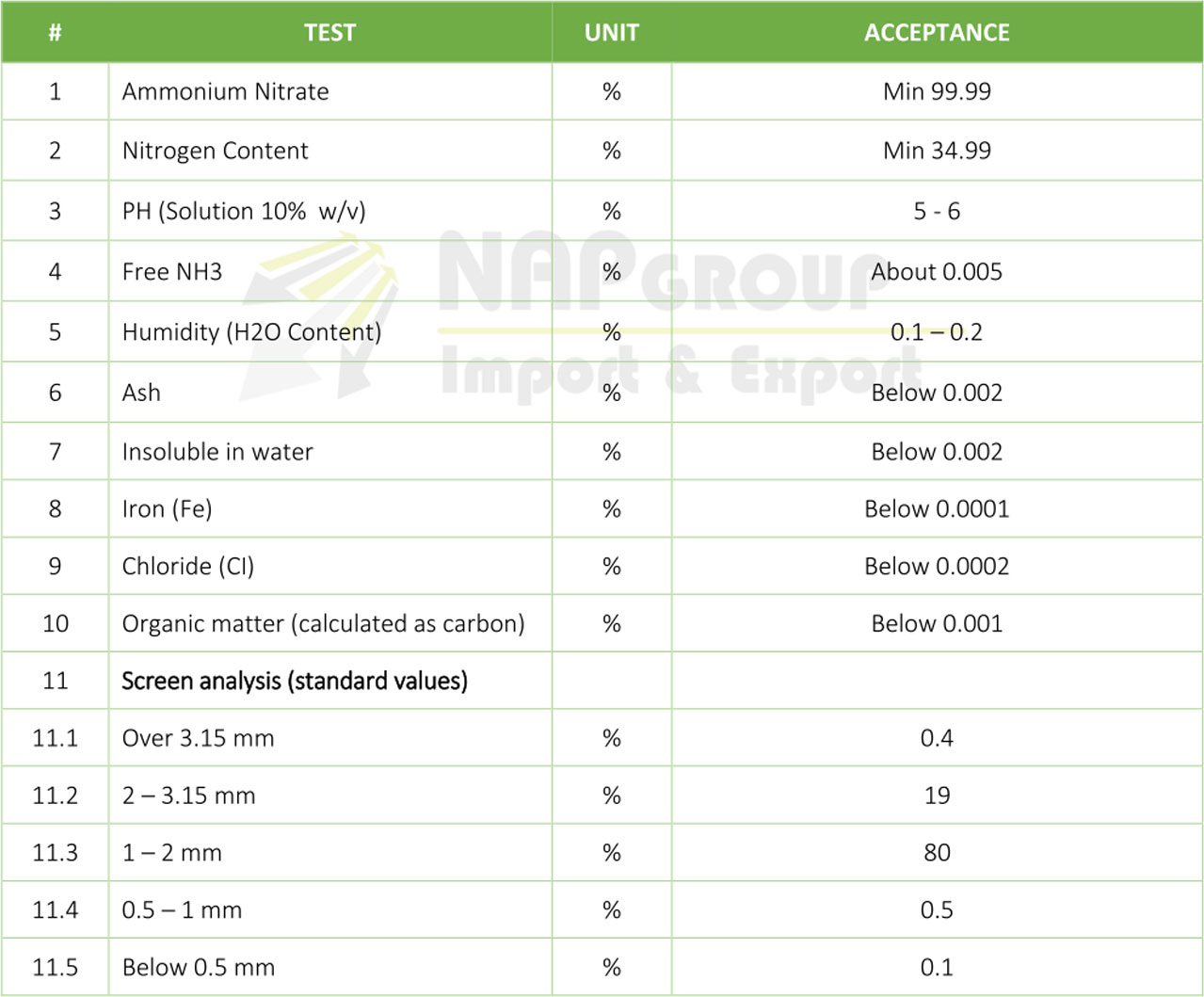
– Ammonium nitrate is also used in instant cold packs. In this use, ammonium nitrate is mixed with water in an endothermic reaction, which absorbs 26.2 kilojoules of heat per mole of reactant.
– Products of Ammonium nitrate reactions are used in Airbags. Sodium azide (NaN3) is the chemical used in airbags, as it decomposes to Na (s) and N2 (g).
– Used in the treatment of some titanium ores.
– Used in survival kits mixed with zinc dust and ammonium chloride because it will ignite on contact with water.
– Used as a nutrient in producing antibiotics and yeast.
– Ammonium nitrate can be used to make anhydrous ammonia, a chemical often used in the production of methamphetamine.